Point 4
Growth trajectory
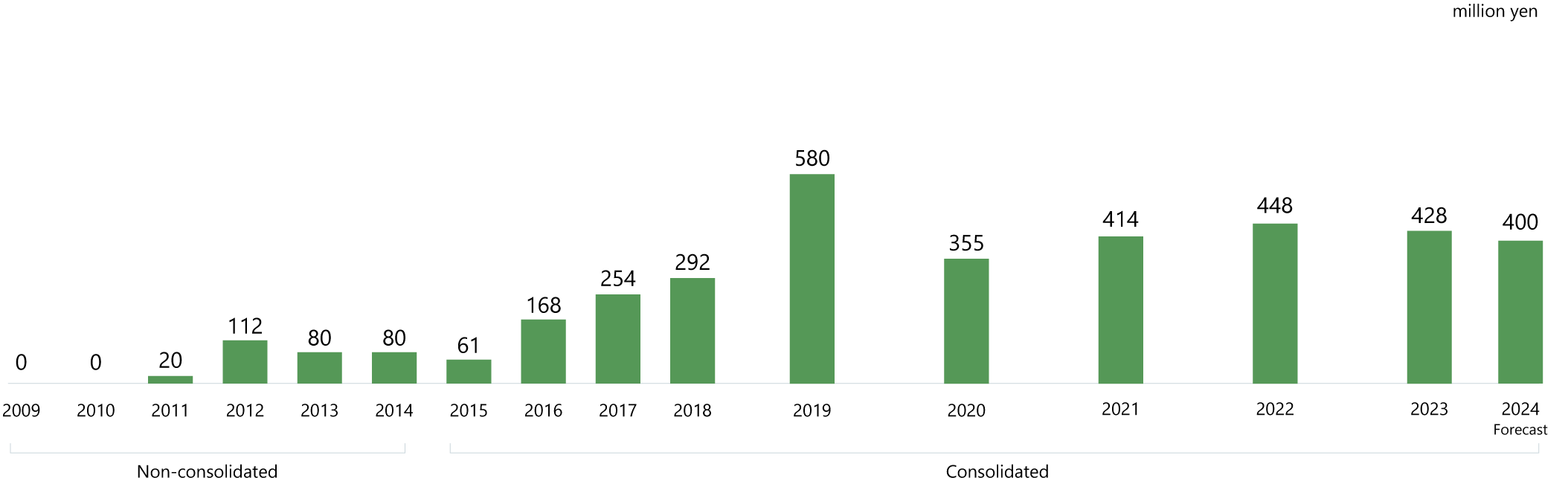
Building a solid earnings base
In the drug discovery business, development requires many years and substantial investment. So, in addition to product sales, the progress of our pipeline—the source of future earnings—is an important factor for growth.
DWTI is making steady progress in the development of its pipeline drugs.
Sales
- DW-1001 and DW-1002(Japan) licensed out respectively.
- Ripasudil hydrochloride hydrate[GLANATEC® ophthalmic solution 0.4%] (Korea) and DW-1002(U.S.) approved of new drug application respectively.
- K-321(Fuchs endothelial corneal dystrophy) : Phase 2 clinical trials (U.S.) started.
- DW-1002 launched in U.S..
- Ripasudil hydrochloride hydrate[GLANATEC® ophthalmic solution 0.4%] (3 countries in Asia) approved of new drug application.
- DW-1002 launched in Canada.
- K-232(Ripasudil hydrochloride hydrate and Brimonidine tartrate fixed combination eye drop) application filed in Japan.
- DW-1001 started phase I clinical trials in Japan.
- DWR-2206 (Regenerative medicine cell-product) signed a joint development agreement with ActualEyes Inc..
- K-321 (Fuchs endothelial corneal dystrophy) started phase III clinical trials in U.S..
- “GLA-ALPHA® combination ophthalmic solution” launched in Japan.
- H-1337(Glaucoma and ocular hypertension) started phase IIb clinical trials in U.S..
- DW-1002 (Single drug) application filed in China, DW-1002 (Combination drug) decided to develop in U.S..
- K-321 (Fuchs endothelial corneal dystrophy) started global phase III clinical trials in U.S..
A number of products of pipeline
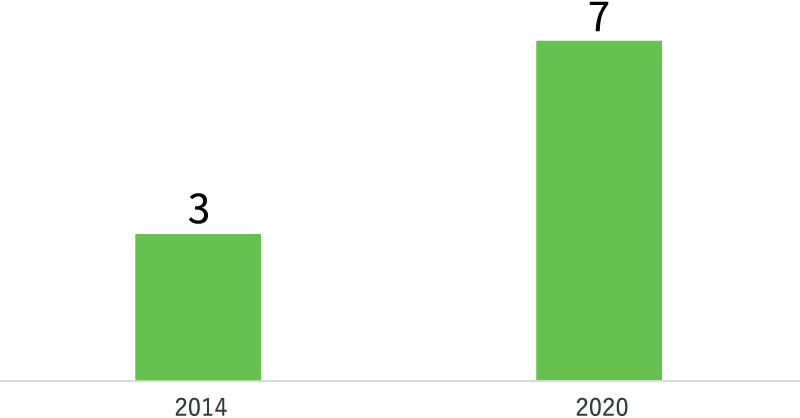
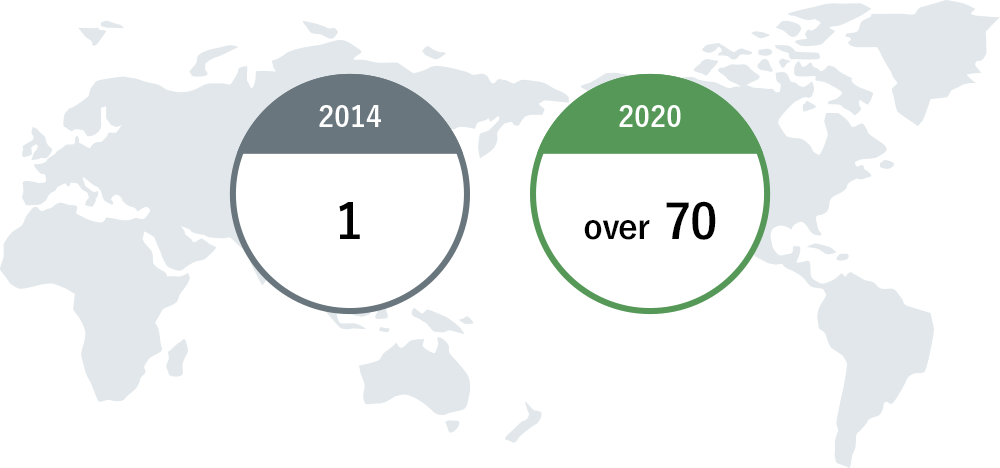
Countries / Areas where products on the market are sold