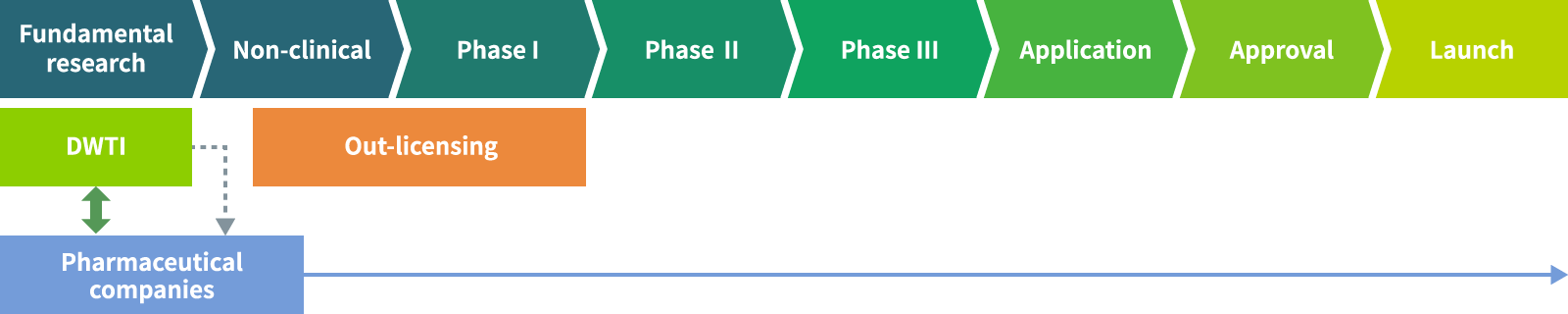
Our business model

Drug development begins with basic research and goes through nonclinical studies and clinical trials (Phase I, II, and III) before applying for approval. Once approved, the drug is launched.
DWTI is concentrating its management resources on the upstream of new drug development, from basic research through to the clinical development phases.
The results obtained from these R&D activities (new drug candidates) are licensed out to pharmaceutical or other biotech companies.
Our sales derive mainly from the following:
1. Upfront payment received at the time of out-licensing
2. Milestone payments received when each development milestone is reached in the progress of clinical trials
3. Royalties, a certain percentage of sales of a post-launch drug
About our pipeline
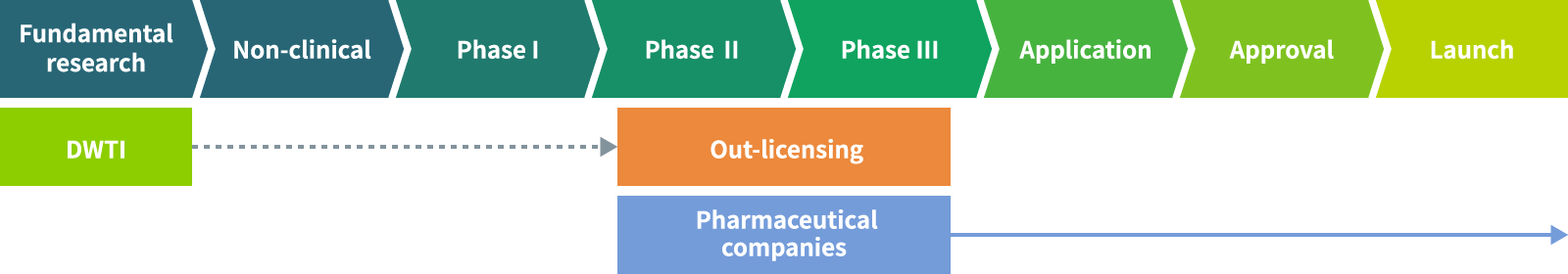
DWTI’s drug discovery, development, and out-licensing process
In-house products discovery and development
Discover drug seeds using proprietary basic technology and develop them to a certain stage
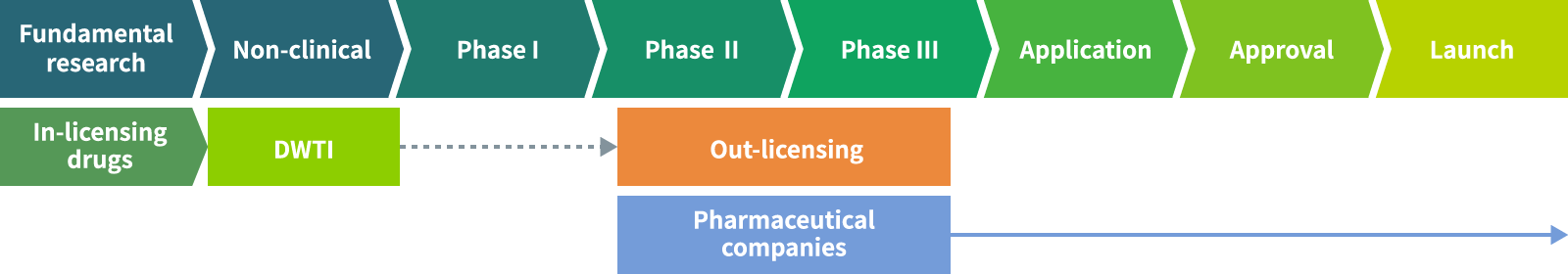
Development of in-licensed products
In-licensed products developed by others and develop them to a certain stage
Collaborative drug discovery
Discover new drugs in collaboration with third parties, which the third parties then proceed with development